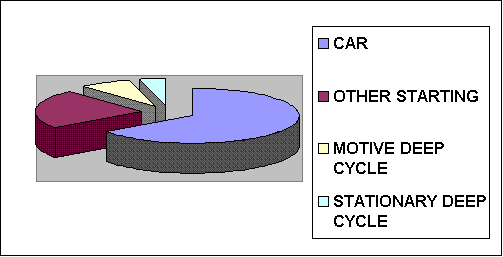
A lead-acid battery (also know as an "accumulator") is a secondary (rechargeable) electrochemical device that stores chemical energy and releases it as electrical energy upon demand. When a battery is connected to a resistive load, such as a motor, chemical energy is converted to electrical energy and direct current flows through the circuit. Approximately 1.3 billion lead-acid batteries are in use worldwide in 2016 with starting batteries representing approximately 88% of the total. The total breaks down to 65% Car, 23% Other Starting Batteries (motorcycle, etc.), 8% Deep Cycle Motive (wheelchairs, golf carts, fork lift trucks, etc.), and 4% Deep Cycle stationary (backup, UPS, standby, etc.). Lead-acid batteries consume 80% of all the lead that produced or recycled.
BATTERY PRODUCTION

In the order of importance, the four major purposes of a car or "SLI" (Starting, Lighting and Ignition) battery, as it is known in the battery industry, are:
A good quality wet car battery will cost between $90 and $125 and, if properly maintained, should last five years or more. In 1927, a car battery typically cost $70. With an estimated 3% compounded annual growth rate, worldwide retail sales of car lead-acid batteries represent roughly 63% of the estimated $30 billion annually spent on batteries. In North America, BCI reports that approximately 120 million car and motorcycle batteries were sold in 2010, of which approximately 88% were for replacement and 12% were for original equipment. For 2003, Eurobat estimates that in Western Europe 58.5 million car batteries will be sold and 71% will be replacement (after market) and 29% will be OEM (Original Equipment Manufacturer). In 2016, Johnson Controls is the largest global manufacturer with 33% of the approximately 438 million annually produced. This is followed by Yuasa and Exide. In another marketing study by Recharge, in 2003 the worldwide battery market was roughly $30 billion, with 30% of that being SLI (car) and 15.3% industrial (deep cycle) lead-acid batteries. In the June 2015 report, Environmental Protection Agency stated that approximately 99% of all lead-acid car batteries were recycled in the U.S. in 2013, making lead one of the highest reclaimed non-precious metals. Steel cars at 70.6% was a distant second.
The purpose of a deep cycle battery is to provide electric power for wheelchairs, trolling motors, golf carts, boats, fork lift trucks, uninterruptible power supplies (UPS), and other accessories for marine and recreational vehicle (RV), commercial and stationary applications. A good quality wet deep cycle (or "leisure") battery will cost between $100 and $300 and, if properly maintained and used, will give you at least 200 deep discharge-charge cycles. For differences between a car and deep cycle battery, please see Section 7.1.8. Purportedly, Exide and EnerSys are the two largest deep cycle battery manufacturers in the world.
A 12-volt lead-acid battery is made up of six cells, each cell producing approximately 2.11 volts that are connected in series from POSITIVE (+) terminal of the first cell to the NEGATIVE (-) terminal of the second cell and so on. Each cell is made up of an element containing positive plates that are all connected together and negative plates, which are also all connected together. They are individually separated with thin sheets of electrically insulating, porous material "envelopes" or "separators" (in the diagram below) that are used as spacers between the positive (usually light orange) and negative (usually slate gray) plates to keep them from electrically shorting to each other. The plates (in the diagram below), within a cell, alternate with a positive plate, a negative plate and so on.
CAR BATTERY CONSTRUCTION
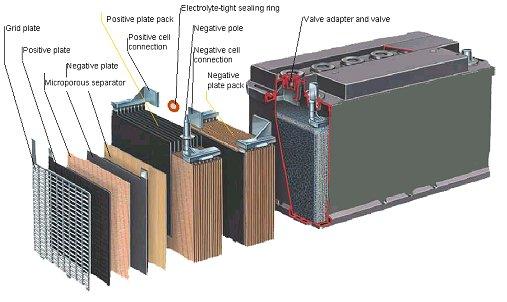
[Source: Eurobat]
DEEP CYCLE BATTERY CONSTRUCTION
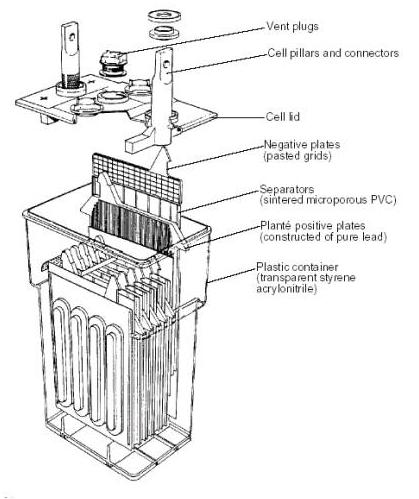
[Source: US Department of Energy]
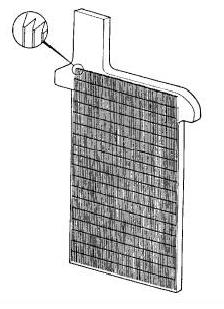
The most common plate type in use today is made up of a metal grid that serves as the supporting framework for the active porous material that is "pasted" on it. After the "curing" of the plates, they are made up into cells, and the cells are inserted into a high-density tough polypropylene or hard rubber case. The positive plates in cells are connected in parallel to the external POSITIVE (+) terminal and the negative plates in each cell are connected to the NEGATIVE (-) external terminal. Instead of pasted Lead Oxide, some batteries are constructed with more expensive solid lead cylindrical (spiral wound); Manchester or "Manchex" (buttons inserted into the grid); tubular; or prismatic (flat) solid lead (Planté) positive plates. The case is covered and then filled with a dilute sulfuric acid electrolyte. The battery is initially charged or "formed" to convert the active yellow Lead Oxide (PbO or Litharge) in the positive plates (cathode) into Lead Peroxide (PbO2), which is usually dark brown or black. The active material in the negative pasted plates (anode) becomes sponge Lead (Pb), but with a very porous structure which is slate gray. If sponge Lead is rubbed with a hard object, it will be silvery in color. Antimony (Sb), Calcium (Ca) or other alloys are often added to the plates to enhance their performance or service life. The electrolyte is replaced and the battery is given a finishing charge. A "Wet charged" battery is a wet lead-acid battery shipped with electrolyte in the battery and a "dry charged" battery is shipped without electrolyte. When dry charged batteries are sold, electrolyte (battery acid) is added, allowed to soak into the plates, charged (or "formed"), and put into service. This avoids having to maintain the batteries until they are sold.
PASTED PLATE
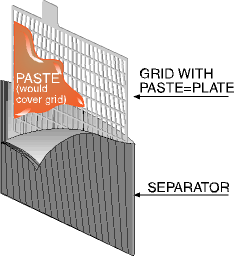
[Source: BCI]
FLAT AND TUBULAR PLATE
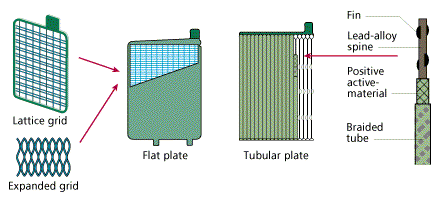
PLANTÉ PLATE
[Source: US Department of Energy]
SPIRAL WOUND PLATE
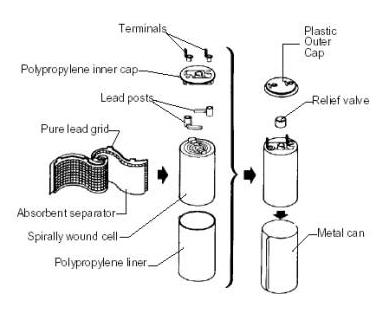
[Source: US Department of Energy]
Two important considerations in battery construction are porosity and diffusion. Porosity is the pits and tunnels in the plate that allows the sulphuric acid to get to the interior of the plate. Diffusion is the spreading, intermingling and mixing of one fluid with another. When you are using your battery, the fresh acid needs to be in contact with the plate material and the water generated needs to be carried away from the plate. The larger the pores or warmer the electrolyte, the better the diffusion.
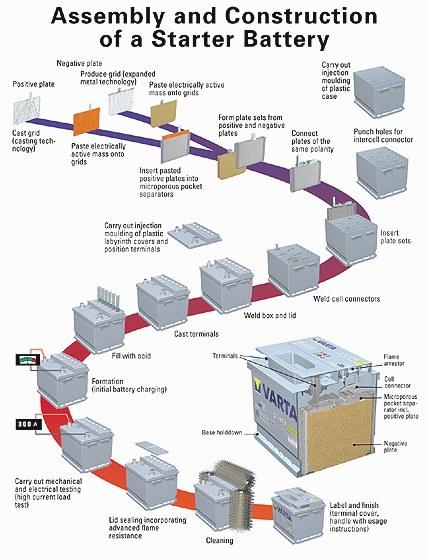
[Source: Varta]
There is an excellent detailed description of how battery is made, equipment used and quality assurance on the Best Manufacturing Practices Web site at http://www.bmpcoe.org/library/books/navso%20p-3676/index.html/. If you prefer watching a Quick Time video, Surrette has a first-class one on http://www.surrette.com/rolls/video/video.htm/.
DISCHARGING PROCESS
PbO2 + Pb + 2H2SO4 → 2PbSO4 + H2O
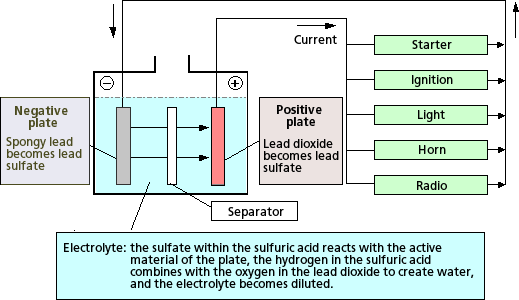
CHARGING PROCESS (Reverse of Discharging Process)
2PbSO4 + H2O → PbO2 + Pb + 2H2SO4
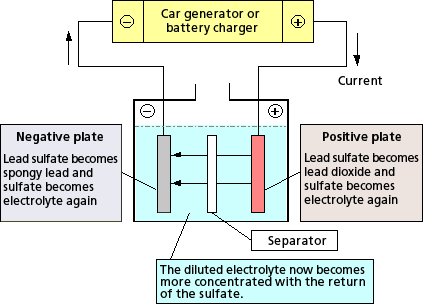
[Source: Battery Association of Japan]
A battery is created by electrochemical reaction of alternating two different metals such as Lead Dioxide (PbO2), the positive plates, and Sponge lead (Pb), the negative plates immersed in diluted Sulfuric Acid (H2SO4), electrolyte. The types of metals and the electrolyte used will determine the output of a cell. A typical fully charged lead-acid battery produces approximately 2.11 volts per cell. The chemical action between the metals and the electrolyte (battery acid) creates the electrical energy. Energy flows from the battery as soon as there is an electrical load, for example, a starter motor, that completes a circuit between the positive terminal connected to the positive plates and the negative terminal connected to the negative plates. Electrical current flows as charged portions of acid (ions) between the battery plates and as electrons through the external circuit. The action of the lead-acid storage battery is determined by chemicals used, State-of-Charge, temperature, porosity, diffusion, and load. A cycle is defined as one discharge and one recharge of the battery.
A more detailed description of how a battery works can be found on the BCI web site at http://batterycouncil.org/?page=Lead_Acid_Batteries#howworks.
When the active material in the plates can no longer sustain a discharge current, a battery "dies". Normally a car (or starting) battery "ages" as sulfation builds up on the negative plates or the active positive plate material sheds (or flakes off) due to the normal expansion and contraction that occurs during the discharge and charge cycles. This causes a loss of plate performance or capacity. A brown sediment, called sludge or "mud," that builds up in the bottom of the case and can short the plates of a cell out. This will kill the battery as soon as the short occurs. In hot climates, additional causes of failure are positive grid growth, positive grid metal corrosion, Dendrite formation, negative grid shrinkage, buckling of plates, or loss of water. Deep discharges, heat, vibration, fast charging, and overcharging all accelerate the "aging" process. Approximately 50% of premature car battery failures is caused by the loss of water for normal recharging charging due to the lack of maintenance, evaporation from high under hood heat, or overcharging. Positive grid growth and undercharging causing sulfation and electrical system malfunctions also cause premature battery failures.
Normally, well maintained and properly charged deep cycle batteries naturally die due to positive grid corrosion causing an open connection. The shedding of active material is an additional cause. If deep cycle battery is left discharged for long period of time, dendrite shorts between the plates can occur when the battery is recharged. The low resistance bridge in the shorted cell will heat up and boil the electrolyte out of the cell causing a high volumes of hydrogen and oxygen. That is why proper venting and ventilation is so important when recharging batteries. Approximately 85% of premature deep cycle and starting batteries failures that are not recharged on a regular basis is due to an accumulation of sulfation. Sulfation is caused when a battery's State-of-Charge drops below 100% for long periods or under charging. Hard lead sulfate crystals fills the pours and coats the plates. Please see Section 16 for more information on sulfation. Recharging a sulfated battery is like trying to wash your hands with gloves on.
In a hot climate, the harshest environment for a battery, a Johnson Controls survey of junk batteries revealed that the average life of a car battery was 37 months. In a separate North American study by BCI, the average life was 48 months. In a study by Interstate Batteries, the life expectancy in extreme heat was 30 months. If your car battery is more than three years old and you live in a hot climate, then your battery is probably living on borrowed time. Abnormally slow cranking, especially on a cold day, is another good indication that your battery is going bad. It should be externally recharged, surface charge removed, and load tested. Dead batteries almost always occur at the most inopportune times. You can easily spend the cost of a new battery or more for an emergency jump start, tow or taxi ride.
Most of the "defective" batteries returned to manufacturers during free replacement warranty periods are good. This strongly suggests that some sellers of new batteries do not know how to or fail to take the time to properly recharge and test batteries. This situation is improving with the widespread use of easy to use battery testers like those made by Cadex, Midtronics and Solar. They are used to predict the CCA performance or Amp Hour/Reserve capacity of the batteries.