[Source: Exide]
Performing preventive maintenance on wet batteries is easy and should occur at least once a month during the summer and every three months during the rest of the year, especially in hot climates. While working with car and deep cycle lead-acid batteries, please always wear safety goggles or glasses to protect your eyes in case of a battery explosion. Here are some simple steps to maintain your battery:
3.1. Before you start the engine for the first time during the day, check the the State-of-Charge (SoC) of the battery. For non-sealed (with filler caps) wet batteries, check the electrolyte levels in each cell. Please see Section 4.4 for more information on determining the SoC. If the battery is not fully charged (100% State-of-Charge), recharge it with a battery charger in a well ventilated area. Please see Section 9 for more information on charging. This is because State-of-Charge is based on your driving habits. Some vehicle charging systems have been known to undercharge the battery causing an accumulation of lead sulfate, known as sulfation. A gradual build up of sulfation will reduce the performance and capacity of the battery. Please see Section 16 for more information on sulfation. Periodically fully recharging or "topping off" with a battery charger will restore most or all of the battery's capacity. At least every one to three months is recommended depending on temperature and driving habits.
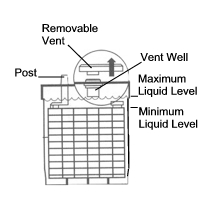
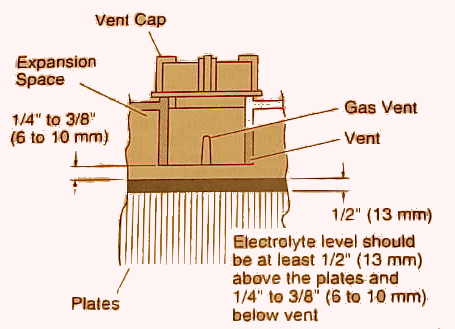
3.2. Checking electrolyte levels of non-sealed (with filler caps) wet batteries every three months is recommended. In hot climates, checking the electrolyte levels at least once a month during the summer is recommended. The plates need to be covered at all times to prevent sulfation and reduce the possibility of an internal battery explosion. For non-sealed wet car and small deep cycle batteries (less than 200 amp hours), allow the battery to cool to room temperature. Then add only distilled water to the level indicated by the battery manufacturer, UPPER LEVEL mark, or just to the bottom of the filler tubes (vent wells or splash barrels) as shown in the diagram below. For large deep cycle batteries, fill to within 1/4 to 3/8 inch (6 to 10 mm) below the bottom of the filler tubes. Avoid overfilling, especially in hot weather, because the heat will cause the electrolyte to expand and overflow. In an emergency, use rain water. Do not use tap water or water from residential Reverse Osmosis (RO) systems to refill batteries because it could contain chlorine, calcium or magnesium and produce calcium or magnesium sulfate crystals. These crystals can gradually fill the pores or coat the plates which will reduce the battery's capacity. State-of-Charge (SoC) readings will be inaccurate immediately after the addition of water, recharges or discharges. Please see Section 4.3 for more information on removing a Surface Charge.
ELECTROLYTE FILL LEVELS FOR SMALL BATTERIES
Less Than 200 AH

[Source: Exide]
ELECTROLYTE FILL LEVELS FOR LARGER BATTERIES
Greater Than 200 AH
[Source: Mountain Top Golf Cars]
To reduce the water consumption for wet batteries, there are battery caps, for example, Hydocaps, Water-Mizer, etc. that are specifically designed for that purpose. To automatically water batteries, there are watering systems that will add water to each individual battery cell. Please see Watering Systems and Battery Filler Caps for more information. If electrolyte has been spilled, please see Section 9.14 for more information on adding electrolyte or adjusting the Specific Gravity within a cell. If a battery has dried out due to an overcharge, you can try to recovery it by refilling with distilled water and slowly recharging it. It might take several discharge/charge cycles before some of the capacity is restored.
3.3. For safety, wear eye protection. Remove any corrosion, oxidation, paint or rust with a brass wire battery brush or with a "ScotchBrite" pad from the terminal's connecting surfaces on both ends of each battery cables, battery posts, lugs or terminals, and engine ground strap connections. Bare metal to metal is necessary for very low electrical resistance and good current conductivity. Disconnect the negative terminal first, and brush the corrosion away from you. A stiff steel wire brush or sandpaper may damage protective lead plating on copper connectors or terminals. Corrosion is normally a white powdery lead oxide substance, but could have other colors mixed in like gray, yellow or green from other chemicals or alloys. Heavy corrosion can be neutralized by applying a thin paste from a mixture of baking soda (bicarbonate of soda) and warm water. Some use diet cola to dissolve corrosion because it does not contain sugar which will leave a sticky surface. Wash off the residue with clean water and dry. Take care not to contaminate the battery's electrolyte through the filler or vent holes.
Terminal corrosion/oxidation is one of the leading causes for a engine not to start or other electrical system problems. It prevents the battery from receiving a charge or providing high current to the starter due to a high resistance connection, which eventually causes the battery to be drained or voltage loss. Some vehicle manufacturers, BMW and VW/Audi for example, are using a Battery Management Systems (BMS) is some vehicles that require battery identification, so disconnecting battery terminals for cleaning might disable the charging system. Please check online, service manual, owner's manual or dealer before disconnecting a battery terminal to determine if an OEM (Original Equipment Manufacturer) battery required or if a special OBD scanning tool is necessary to reprogram or reactivate the BMS. For more information on dealing with vehicles with BMS or saving the emission computer setting, please see Section 8.
To prevent terminal corrosion, thinly coat the terminals, posts, clamps, lugs and exposed metal around the battery with dielectric grease, high temperature wheel bearing grease, lithium grease, silicone, or a commercial battery terminal protector. Petroleum jelly ("Vaseline") or Calcium grease is not recommended for use under the hood because it has a low melting point. Gluing a sacrificial anode, such as a solid piece of copper to the top of the battery between the posts or using sealed VRLA batteries will prevent or reduce terminal corrosion. Please do not use the felt or metal washers between the mating conductive surfaces with General Motors-type side, "L" or threaded stud terminals as shown in Section 7.5. This is because a large cross section of conductive area is required to carry the heavy starting current. For batteries not subject to high temperatures, use "No Oxide A" (or the battery manufacturer's recommended coatings) on the terminal posts, clamps, lugs, or connectors. Some stainless steel alloys and other metal clamps, lugs, washers, nuts and bolts have also been known to cause problems with electrolysis and high resistance.
Corrosion/oxidation is caused by one or more the following:
3.4. Tighten loose battery terminal clamps, lugs, terminals, connectors, and hold-down clamps to prevent excessive vibration. Use of Nyloc self locking nuts will help prevent loose hold-down clamps.
3.5. Clean the battery top to eliminate conductive paths created by dried or wet electrolyte and to prevent corrosion.
3.6. Clean the charging system to allow better heat transfer and check the alternator belts for cracks and correct tension.
3.7. Replace any battery cables (or cable terminals) that are corroding, swelling or damaged with an equal or larger diameter cable. If electrical problems are experienced in vehicles with GM's side terminal connectors, check for corrosion inside the positive terminal, lug or connector with the multiple cables. Larger cable and lugs, connectors or terminals are better because there is more surface area and less voltage drop.
3.8. Replace the battery if the battery case is bulging, cracked or leaking, especially around "GM" style side terminals.
3.9. Periodically rotate batteries in a bank of deep cycle batteries because the lowest capacity batteries tend to fail first and to insure that the connections are clean and tight.