[Source: Andre Packwood]
4.4. Measure State-of-Charge (SoC)
Electrolyte Freeze Points Table
4.4.4. Interpreting the SoC Measurements
4.5. Performance or Capacity Load Testing
While working with car or deep cycle lead-acid batteries, please help to prevent blindness by always wearing safety glasses (or glasses) in the event of an explosion. Below are eight simple steps in performance and capacity testing a battery. Alternatively, some auto parts or battery stores in the United States and Canada, like Auto Zone, Sears, Wal-Mart, Pep Boys, etc., will test your battery, charging system and starter for free. If you have a non-sealed wet battery (with filler caps), it is highly recommended that you use a good quality temperature compensating hydrometer, like an E-Z Red SP101, which can be purchased online or at an auto parts or battery store for less than $10, or a refractometer for less than $50.
If you have a sealed battery or need to troubleshoot a charging or electrical system, you will need a digital voltmeter with 0.5% (or better) DC accuracy. A digital voltmeter (or multimeter) can be purchased at an electronics store for between $30 and $300. Analog voltmeters are not accurate enough to measure the millivolt differences of a battery's State-of-Charge or output of the charging system. Do not use a 12-volt test light to troubleshoot vehicle electrical circuits, except for testing the parasitic load at the battery, because you might damage the emissions computer or other sensitive electronic devices.. A battery performance load tester is optional. For batteries with at least a 50% State-of-Charge, another way of testing the CCA (Cold Cranking Amp) starting performance or Reserve Capacity (RC) or amp hour (AH) capacity of lead-acid batteries is using an electro-chemical impedance spectroscopy (EIS) tester, such as a Cadex Spectro or a conductance tester, for example a Midtronics.
A sulfated sealed battery's voltage often will read higher than the SoC actually is, so load testing maybe required to determine the battery's actual performance or capacity.
Inspect for obvious problems such as a low electrolyte levels; loose, corroded or swollen cables, corroded battery terminals or posts; loose or broken alternator belt; frozen battery; loose hold-down clamps; dirty or wet battery top; or a leaking, cracked, bulging or damaged battery case or terminals. If the electrolyte levels are below the tops of the plates, add enough distilled, deionized or demineralized water to cover the plates and recharge the battery, allow to cool to room temperature and then top off the levels. The plates need to be covered at all times to prevent sulfation and reduce the possibility of an internal battery explosion. Please see Section 3.2 for electrolyte fill level information.
If electrolyte has been spilled, please see Section 9.14 for more information on adding electrolyte or adjusting the Specific Gravity within a cell.
Charge the battery to 100% State-of-Charge in a well ventilated area. If a non-sealed wet battery has a .030 (sometimes expressed as 30 "points") or more difference in Specific Gravity reading between the lowest and highest cell or if a cell is .010 or 10 "points" below the reading for a fully charged cell, then you should equalize the battery using the battery manufacturer's procedures. (Please see Section 9.1.4 for more information on equalize charging.)
Surface charge (or "counter voltage") is the uneven mixture of sulfuric acid and water along the surface of the plates as a result of charging or discharging as the electrolyte has an opportunity to diffuse in the pores of the plates. It will make a weak battery appear good or a good battery appear bad. Larger wet lead-acid batteries (especially over 100 amp hours) could also have electrolyte stratification where the concentration of acid is greater at the bottom of the cell than near the surface. The Open Circuit Voltage (OCV) will read higher than they actually are. Stratification can be eliminated by an equalizing charge, stirring or gently shaking the battery to mix the electrolyte.
A surface charge can be eliminated by one of the following methods after recharging a lead-acid battery:
4.4. Measure the State-of-Charge (SoC)
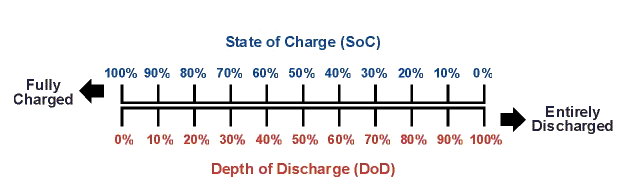
The State-of-Charge acts like a "battery fuel gauge", but it only measures the state of the battery's charge and not its storage capacity, or state of health to produce rated starting current or performance. For storage capacity measurements, please see Section 4.5, below. For example, a 50% SoC reading does not necessarily mean that a 100 amp hour (C/20) battery will produce 50 amp hours at five amp discharge load (with five amps being the 20 hour discharge load) or starting current. This is because the battery might not have 100 amp hours of storage capacity to begin with. Depth-of-Discharge (DoD) is the inverse of State-of-Charge (SoC) as shown in the following graphic.

[Source: Andre Packwood]
To measure a battery's State-of-Charge, perform the following steps:
A downloadable Temperature Compensated Battery State-of-Charge (SoC) Table is available. When printed, this Excel spreadsheet produces a single page that contains a table with the Specific Gravity and Open Circuit Voltage measurements by temperature vs. various States-of-Charges. This table is for wet Low Maintenance (Ca/Sb), wet Standard (Sb/Sb), and wet "Maintenance Free" (Ca/Ca) or VRLA (AGM or Gel Cell) batteries. The file size is approximately 22 KBytes.
4.4.1. Specific Gravity vs. Temperature at Various States-Of-Charge (SoC) for a Wet Low Maintenance (Sb/Ca) or Standard (Sb/Sb) Battery Table
Using a temperature compensated hydrometer (or refractometer) to measure the Specific Gravity is the most accurate way of determining a wet, non-sealed (with filler caps) lead-acid battery's SoC. When the SoC measured by a hydrometer (or refractometer) does not materially agree with the SoC measured by an accurate digital voltmeter, it is probably due to sulfation. If you suspect that a battery is sulfated, it probably is, especially if it will not hold a charge, has not been charged in a while, or has been continuously undercharged. For more on sulfation, please see Section 16. This table has a baseline that assumes that a 1.265 Specific Gravity (SG) reading for a fully charged (100% SoC), wet Low Maintenance (Sb/Ca) or Standard (Sb/Sb) lead-acid battery at rest at 77° F (25° C). The Specific Gravity readings for a battery at 100% SoC will vary by plate chemistry, so if possible, check the battery manufacturer's specifications for their State-of-Charge definitions for the battery being measured. If the baseline is unknown at 100% SoC, please see Section 9.5. How Do I Know When My Battery Is Fully Charged? Depending on the plate chemistry, the Specific Gravity can range from 1.215 to 1.300 for a fully charged wet Low Maintenance (Sb/Ca) or Standard (Sb/Sb) car batteries at 77° F (25° C) and tend to be higher in deep cycle batteries.
Specific Gravity vs. Temperature
at Various States-Of-Charge (SoC)
for a Wet Low Maintenance (Sb/Ca)
or Standard (Sb/Sb) Battery Table
|
Electrolyte Temperature (Fahrenheit) |
Electrolyte Temperature (Celsius) |
100% SoC |
75% SoC |
50% SoC |
25% SoC |
0% SoC |
|
120° |
48.9° |
1.249 |
1.209 |
1.174 |
1.139 |
1.104 |
|
110° |
43.3° |
1.253 |
1.213 |
1.178 |
1.143 |
1.108 |
|
100° |
37.8° |
1.257 |
1.217 |
1.182 |
1.147 |
1.112 |
|
90° |
32.2° |
1.261 |
1.221 |
1.186 |
1.151 |
1.116 |
|
77° |
25° |
1.265 |
1.225 |
1.190 |
1.155 |
1.120 |
|
70° |
21.1° |
1.269 |
1.229 |
1.194 |
1.159 |
1.124 |
|
60° |
15.6° |
1.273 |
1.233 |
1.198 |
1.163 |
1.128 |
|
50° |
10.0° |
1.277 |
1.237 |
1.202 |
1.167 |
1.132 |
|
40° |
4.4° |
1.281 |
1.241 |
1.206 |
1.171 |
1.136 |
|
30° |
-1.1° |
1.285 |
1.245 |
1.210 |
1.175 |
1.140 |
|
20° |
-6.7° |
1.289 |
1.249 |
1.214 |
1.179 |
1.144 |
|
10° |
-12.2° |
1.293 |
1.253 |
1.218 |
1.183 |
1.148 |
|
0° |
-17.8° |
1.297 |
1.257 |
1.222 |
1.187 |
1.152 |
For example, if the electrolyte is at 20° F (-6.7° C), the Specific Gravity reading would be 1.289 for a 100% State-of-Charge because the liquid is more dense at the colder temperature. At 100° F (37.8° C), the Specific Gravity reading would be 1.182 for 50% SoC and a reading of 1.104 or lower at 120° F (48.9° C) would indicate a discharged battery.
Specific Gravity vs. Temperature
at Various States-Of-Charge (SoC)
for a Wet Non-Sealed "Maintenance Free" (Ca/Ca) Battery Table
|
Electrolyte Temperature (Fahrenheit) |
Electrolyte Temperature (Celsius) |
100% SoC |
75% SoC |
50% SoC |
25% SoC |
0% SoC |
|
120° |
48.9° |
1.264 |
1.224 |
1.189 |
1.154 |
1.119 |
|
110° |
43.3° |
1.268 |
1.228 |
1.193 |
1.158 |
1.123 |
|
100° |
37.8° |
1.272 |
1.232 |
1.197 |
1.162 |
1.127 |
|
90° |
32.2° |
1.276 |
1.236 |
1.201 |
1.166 |
1.131 |
|
77° |
25° |
1.280 |
1.240 |
1.205 |
1.170 |
1.135 |
|
70° |
21.1° |
1.284 |
1.244 |
1.209 |
1.174 |
1.139 |
|
60° |
15.6° |
1.288 |
1.248 |
1.213 |
1.178 |
1.143 |
|
50° |
10.0° |
1.292 |
1.252 |
1.217 |
1.182 |
1.147 |
|
40° |
4.4° |
1.296 |
1.256 |
1.221 |
1.186 |
1.151 |
|
30° |
-1.1° |
1.300 |
1.260 |
1.225 |
1.190 |
1.155 |
|
20° |
-6.7° |
1.304 |
1.264 |
1.229 |
1.194 |
1.159 |
|
10° |
-12.2° |
1.308 |
1.268 |
1.233 |
1.198 |
1.163 |
|
0° |
-17.8° |
1.312 |
1.272 |
1.237 |
1.202 |
1.167 |
For example for a wet "Maintenance Free" battery, if the electrolyte is at 20° F (-6.7° C), the Specific Gravity reading would be 1.304 for a 100% State-of-Charge because the liquid is more dense at the colder temperature. At 100° F (37.8° C), the Specific Gravity reading would be 1.197 for 50% SoC and a reading of 1.119 or lower at 120° F (48.9° C) would indicate a discharged battery.
HOW DO I USE A HYDROMETER? A hydrometer is an inexpensive float-type device used to measure the concentration of sulfuric acid (Specific Gravity) of battery electrolyte ("battery acid"). From this reading you can easily and accurately determine a non-sealed battery's State-of-Charge. A hydrometer is a glass barrel or plastic container with a rubber nozzle or hose on one end and a soft rubber bulb on the other. Inside the barrel or container, there is a float and calibrated graduations used for the Specific Gravity measurement. The following is a list of instructions on how to correctly use a battery hydrometer: BATTERY HYDROMETERS
|
Electrolyte Freeze Points
at Various States-of-Charge
for a Wet Lead-Acid Battery Table
|
Approximate |
Approximate |
Approximate Electrolyte Freeze Point |
|
100% |
0% |
-77°F |
|
75% |
25% |
-35°F |
|
50% |
50% |
-10°F |
|
25% |
75% |
5°F |
|
0% |
100% |
20°F |
4.4.2. Open Circuit Voltage vs. Temperature at Various States Of Charge (SoC) for a Wet Low Maintenance (Sb/Ca) or Standard (Sb/Sb) Battery Table
If the battery is sealed, then use an accurate (0.5% or better) digital voltmeter to measure the battery's Open Circuit Voltage (OCV) to determine the SoC. When the SoC measured by a hydrometer (or refractometer) does not materially agree with the SoC measured by a digital voltmeter, it is probably due to sulfation. If you suspect that a battery is sulfated, it probably is, especially if it has not been charged in a while or has been continuously undercharged. For more on sulfation, please see Section 16. This table has a baseline that assumes that a 12.65 Open Circuit Voltage (OCV) reading for a fully charged (100% SoC), wet Low Maintenance (Sb/Ca) or Standard (Sb/Sb) lead-acid battery at rest, 77° F (25° C), and with the negative terminal disconnected. The OCV readings for a battery at 100% SoC will vary by plate chemistry, so if possible, check the battery manufacturer's specifications for their State-of-Charge definitions for the battery being measured. Depending on the plate chemistry, the Open Circuit Voltage can range from 12.22 to 13.00 for a fully charged wet Low Maintenance (Sb/Ca) or Standard (Sb/Sb) battery at 77° F (25° C). Deep Cycle batteries tend to have higher voltages than car batteries.
Open Circuit Voltage (OCV) vs. Temperature
at Various States Of Charge (SoC)
for Wet Low Maintenance (Sb/Ca)
or Standard (Sb/Sb) Battery Table
|
Electrolyte Temperature (Fahrenheit) |
Electrolyte Temperature (Celsius) |
100% SoC |
75% SoC |
50% SoC |
25% SoC |
0% SoC |
|
120° |
48.9° |
12.663 |
12.463 |
12.253 |
12.073 |
11.903 |
|
110° |
43.3° |
12.661 |
12.461 |
12.251 |
12.071 |
11.901 |
|
100° |
37.8° |
12.658 |
12.458 |
12.248 |
12.068 |
11.898 |
|
90° |
32.2° |
12.655 |
12.455 |
12.245 |
12.065 |
11.895 |
|
77° |
25° |
12.650 |
12.450 |
12.240 |
12.060 |
11.890 |
|
70° |
21.1° |
12.643 |
12.443 |
12.233 |
12.053 |
11.883 |
|
60° |
15.6° |
12.634 |
12.434 |
12.224 |
12.044 |
11.874 |
|
50° |
10.0° |
12.622 |
12.422 |
12.212 |
12.032 |
11.862 |
|
40° |
4.4° |
12.606 |
12.406 |
12.196 |
12.016 |
11.846 |
|
30° |
-1.1° |
12.588 |
12.388 |
12.178 |
11.998 |
11.828 |
|
20° |
-6.7° |
12.566 |
12.366 |
12.156 |
11.976 |
11.806 |
|
10° |
-12.2° |
12.542 |
12.342 |
12.132 |
11.952 |
11.782 |
|
0° |
-17.8° |
12.516 |
12.316 |
12.106 |
11.926 |
11.756 |
For example for a wet low maintenance or standard battery, if the electrolyte is at 20° F (-6.7° C), the Open Circuit Voltage reading would be 12.566 for a 100% State-of-Charge. At 100° F (37.8° C), the Open Circuit Voltage reading would be 12.248 for 50% SoC and a reading of 11.903 or lower at 120° F (48.9° C) would indicate a discharged battery.
4.4.3. Open Circuit Voltage vs. Temperature at Various States Of Charge (SoC) for a Wet "Maintenance Free" (Ca/Ca) or VRLA (AGM or Gel Cell) Battery Table
If the battery is sealed, then use an accurate (0.5% or better) digital voltmeter to measure the battery's Open Circuit Voltage (OCV) to determine the SoC. This table has a baseline that assumes that a 12.78 Open Circuit Voltage (OCV) reading for a fully charged (100% SoC), wet "Maintenance Free" (Ca/Ca) battery at rest, 77° F (25° C) with the negative terminal disconnected. The OCV readings for a battery at 100% SoC will vary by plate chemistry, so if possible, check the battery manufacturer's specifications for their State-of-Charge definitions for the battery being measured. Depending on the plate chemistry, the Open Circuit Voltage can range from 12.6 to 13.1 for fully charged wet "Maintenance Free" (Ca/Ca) batteries and tend to be higher in deep cycle than in vehicle with a ITW Pro "Magic Eye", which only measures the State-of-Charge in ONE of its six cells.
"Magic Eye" Built-in Hydrometer
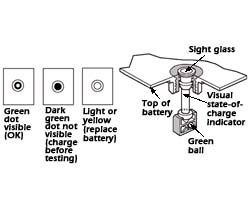
[Source: Popular Mechanics]
Open Circuit Voltage (OCV) vs. Temperature
at Various States-Of-Charge (SoC)
for a Wet "Maintenance Free" (Ca/Ca)
or VRLA (AGM or Gel Cell) Battery Table
|
Electrolyte Temperature (Fahrenheit) |
Electrolyte Temperature (Celsius) |
100% SoC |
75% SoC |
65% SoC |
50% SoC |
25% SoC |
0% SoC |
|
120° |
48.9° |
12.813 |
12.613 |
12.493 |
12.313 |
12.013 |
11.813 |
|
110° |
43.3° |
12.811 |
12.611 |
12.491 |
12.311 |
12.011 |
11.811 |
|
100° |
37.8° |
12.808 |
12.608 |
12.488 |
12.308 |
12.008 |
11.808 |
|
90° |
32.2° |
12.805 |
12.605 |
12.485 |
12.305 |
12.005 |
11.805 |
|
77° |
25° |
12.800 |
12.600 |
12.480 |
12.300 |
12.000 |
11.800 |
|
70° |
21.1° |
12.793 |
12.593 |
12.473 |
12.293 |
11.993 |
11.793 |
|
60° |
15.6° |
12.784 |
12.584 |
12.464 |
12.284 |
11.984 |
11.784 |
|
50° |
10.0° |
12.772 |
12.572 |
12.452 |
12.272 |
11.972 |
11.772 |
|
40° |
4.4° |
12.756 |
12.556 |
12.436 |
12.256 |
11.956 |
11.756 |
|
30° |
-1.1° |
12.738 |
12.538 |
12.418 |
12.238 |
11.938 |
11.738 |
|
20° |
-6.7° |
12.716 |
12.516 |
12.396 |
12.216 |
11.916 |
11.716 |
|
10° |
-12.2° |
12.692 |
12.492 |
12.372 |
12.192 |
11.892 |
11.692 |
|
0° |
-17.8° |
12.666 |
12.466 |
12.346 |
12.166 |
11.866 |
11.666 |
For example for a wet "Maintenance Free" battery, if the ambient temperature is at 20° F (-6.7° C), the Open Circuit Voltage reading would be 12.716 for a 100% State-of-Charge. At 100° F (37.8° C), the Open Circuit Voltage reading would be 12.308 for 50% SoC and a reading of 11.813 or lower at 120° F (48.9° C) would indicate a fully discharged battery.
4.4.4. Interpreting the SoC Measurements
If the State-of-Charge is BELOW 75% using either the Specific Gravity, voltage test or the built-in hydrometer does not indicate "good" (green or blue), then the battery has a low charge and needs to be recharged before proceeding. If the battery is sealed, the battery could have low electrolyte, especially in a hot climate. You should replace the battery, if one of the following conditions occur:
4.5. Performance or Capacity Load Testing
Performance load testing is used to determining a battery's ability to produce current. Capacity load testing is for determining the Reserve Capacity or Amp Hour capacity of a battery. The primarily purpose of a car battery is to start an engine, so the battery's performance (or ability to produce high current) is an important test.
A battery's internal resistance can be computed using the following formula: Internal Resistance = Voltage Drop / Load Current.
4.5.1. Battery's Performance (High Current Method)
If the battery's State-of-Charge is at 75% or higher or has a "good" built-in hydrometer indication, then you can load test the battery by one of the following methods:
DURING the load test, the voltage on a healthy battery will NOT drop below the following table's indicated voltage for the electrolyte at the temperatures shown:
Performance Load Test
|
Electrolyte Temperature Fahrenheit |
Electrolyte Temperature Celsius |
Minimum Voltage Under LOAD |
|
100° |
37.8° |
9.9 |
|
90° |
32.2° |
9.8 |
|
80° |
26.7° |
9.7 |
|
70° |
21.1° |
9.6 |
|
60° |
15.6° |
9.5 |
|
50° |
10.0° |
9.4 |
|
40° |
4.4° |
9.3 |
|
30° |
-1.1° |
9.1 |
|
20° |
-6.7° |
8.9 |
|
10° |
-12.2° |
8.7 |
|
0° |
-17.8° |
8.5 |
[Source: BCI]
4.5.2. Battery Capacity (Low Current Method)
Batteries with Reserve Capacity or Amp Hour capacity ratings can be capacity tested using a slow discharge load test. A DC ammeter and an adjustable resistive load, for example, 12-volt lamps wired in parallel, are required for this test. Please note that this test will not test the battery's performance (ability to produce enough high current to start an engine), but if a battery fails this test, it will probably also fail the high current load capacity test in Section 4.5.1 above.
If the battery is fully charged, the surface charge has been removed, and you know the Reserve Capacity (RC) rating of the battery, then you can test the Reserve Capacity of a battery by applying a constant 25 amp load and discharging the battery to its rated Reserve Capacity in minutes as defined by the battery manufacturer. For example, if you have a 120 minute RC rated battery, then at 80 degrees F (26.7 degrees C) measure the number of minutes it takes to discharge a fully charged battery with a constant 25 amp load to 10.5 volts. Do not discharge the battery below 10.5 volts because you could damage the battery.
If the battery is fully charged, the surface charge has been removed, and you know the Amp Hour rating of the battery, then you can test the capacity of a battery by applying a specific load and discharging the battery to its rated amp hour capacity as defined by the battery manufacturer. Normally the discharge load is the resistance that will discharge a battery in 20 hours (C/20) for car (SLI) and motive deep cycle batteries and eight hours (C/8) for stationary deep cycle batteries. For example, if you have a 100 ampere-hour (C/20) rated battery, then an constant load of five amps would discharge the battery to its rated amp hour capacity in approximately 20 hours (100 AH / 20 Hours = 5 Amps). To determine the capacity, at 80 degrees F (26.7 degrees C) measure the number of hours it takes to discharge a fully charged battery at the discharge rate to 10.5 volts. As the battery discharges, the resistance will have to be decreased to maintain the constant discharge load, at five amps in this example. Do not discharge the battery below 10.5 volts because you could damage the battery.
A battery with 80% or more of its manufacturer's original rated capacity or performance is considered to be good for most applications. Some new batteries can take up to 30 charge/discharge "preconditioning" cycles before they reach their rated capacity. If the battery passed the Capacity Load Test, then skip the next test, Section 4.6 Bounce Back Test and go to Section 4.7. Recharge below.
If the battery has passed the high current performance test, please go to Section 4.7. Recharge below. If not, remove the load, wait ten minutes, and measure the State-of-Charge. If the battery bounces back to less than 75% SoC then recharge the battery (please see Section 9.) and load test again. If the battery fails the load test a second time or bounces back to less than 75% SoC, then replace the battery because it lacks the necessary high current (CCA) performance.
In a well ventilated area, you should recharge your battery to 100% SoC as soon as possible to prevent lead sulfation and to restore it to peak performance.
When the non-sealed wet battery (with filler caps) has cooled to room temperature, recheck the electrolyte levels and, if necessary, fill to the correct levels with distilled water. Please see Section 3.2 for electrolyte fill level information.